The second electron affinity of oxygen is particularly high because the electron is being forced into a small, very electron-dense space. Why is drain-source parasitic capacitance(Cds) omitted in JFET datasheets? This formula would suggest that if we can estimate \(Z_{eff}\), then we can predict the attractive force experienced by, and the energy of, an electron in a multi-electron atom, like Li. This indicates that all electrons in the same shell with a smaller value of l, as well as all electrons in lower shells, shield d and f electrons completely ( n ), due to the poor shielding effect of d electrons. Close Log In. The orbitals must be written based on the increasing energy. This repulsion lessens the attraction the incoming electron feels and so lessens the electron affinity. The inner electrons have less shielding while the outer electrons have more shielding, and hence the effective nuclear charge differs from orbit to orbit. First, the electrons are placed in energy levels further away from the nucleus, which results in electrons not having a strong attraction to the nucleus; secondly, the atom does not want gain electrons because there is minimal charge on the outer energy levels from the nucleus; and lastly, the shielding effect increases, causing repulsion between the electrons, thus they move further from each other and the nucleus itself. If the electron-of-interest is in a d or f subshell, every electron in groups () to the left contributes 1.00 to $\sigma$. Compare trends in \(Z_{eff}\) and atomic size. First Electron Affinity (negative energy because energy released): Second Electron Affinity (positive energy because energy needed is more than gained): When an electron is added to a nonmetal atom, is energy released or absorbed? Notice the negative sign for the electron affinity which shows that energy is released. Why does chlorine have a higher electron affinity than fluorine? What is the reaction that corresponds to the electron affinity of fluorine, F? In fact, while this answer does start off well I have to give it a $-1$ for invoking a concept that does not help and is introduced incorrectly. Estimate the approximate Zeff felt by a valence electron of boron and oxygen, respectively? Trinocular Microscope with DIN Objective and Camera 40x - 2000x, Trinocular Inverted Metallurgical Microscope 100x - 1200x, Junior Medical Microscope with Wide Field Eyepiece & LED 100x - 1500x, Binocular Inverted Metallurgical Microscope 100x - 1200x. 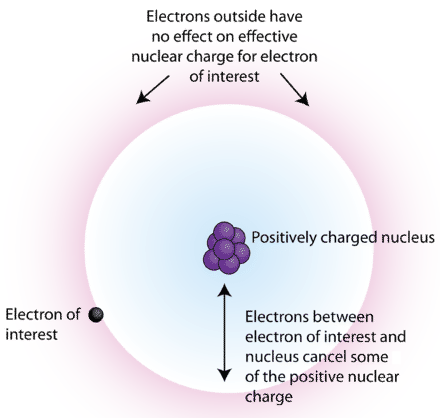 The new shell number is another group. An athlete at the gym holds a 3.0 kg steel ball in his hand. That explanation looks reasonable until you include fluorine! How are common oxidation states divided by this line? or. A chemical reaction that releases energy is called an exothermic reaction and a chemical reaction that absorbs energy is called an endothermic reaction. For example, the effective nuclear charge of magnesium is 3.31 at the periphery while the effective nuclear charge of chlorine is 6.12 at the periphery. If the outermost electrons in cesium experienced the full nuclear charge of +55, a cesium atom would be very small indeed. Down the Group:- As we go down the group of the periodic table, the valence Zeff increases as the atomic number increases down the group. General Chemistry Principles & Modern Applications. Is effective nuclear charge the same for all of the electrons present in an atom? In other words, the \(Z_{eff}\) calculated from Slater's rules are approximate values. Submit. Product was successfully added to your shopping cart. Anions are above the line; cations are below the line. A nuclear charge is equal to the electric charge of a nucleus of an atom. If there is only one electron in an orbit there will be no screening effect taking place. However, all electrons in the same orbit have the same effective nuclear charge. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Greatest --> Least The shielding constant for each group is formed as the sum of the following contributions: If the group (n) is of [s, p] type, an amount of 0.85 from each electron in (n-1)th shell and an amount of 1.00 for each electron from (n-2) and lower shells is added to the shielding constant. Down the table: As we go down a column of the periodic table, the valence \(Z_{eff}\) increases. Therefore, the electrons in oxygen are held closer to the nucleus, giving it a smaller radius. However, one might think that since the number of valence electrons increase going down the group, the element should be more stable and have higher electron affinity. Te The presence of multiple electrons results in more repulsive force and this decreases the nuclear attraction to some extent. Rules for electrons to the left of the group containing electron of interest: If the electron-of-interest is in a d or f subshell, then all electrons in groups to the left of the group having electron-of-interest contribute 1.00 to the shielding constant. Identification of the dagger/mini sword which has been in my family for as long as I can remember (and I am 80 years old), SSD has SMART test PASSED but fails self-testing. This will be always less than the actual nuclear charge due to the shielding effect. What is the ground state electronic configuration of Ti+4? Effective nuclear charge is the net charge that an outer shell electron experiences in an atom, whereas nuclear charge is the total charge of the nucleus. The best answers are voted up and rise to the top, Not the answer you're looking for? You dont consider f orbitals for bromine either. This page titled 1.1.2: Effective Nuclear Charge is shared under a not declared license and was authored, remixed, and/or curated by Kathryn Haas. Thanks to all authors for creating a page that has been read 307,443 times. Explain how and why ionization energy depends on \(Z_{eff}\). A fluorine atom has an electronic structure of 1s22s22px22py22pz1.
The new shell number is another group. An athlete at the gym holds a 3.0 kg steel ball in his hand. That explanation looks reasonable until you include fluorine! How are common oxidation states divided by this line? or. A chemical reaction that releases energy is called an exothermic reaction and a chemical reaction that absorbs energy is called an endothermic reaction. For example, the effective nuclear charge of magnesium is 3.31 at the periphery while the effective nuclear charge of chlorine is 6.12 at the periphery. If the outermost electrons in cesium experienced the full nuclear charge of +55, a cesium atom would be very small indeed. Down the Group:- As we go down the group of the periodic table, the valence Zeff increases as the atomic number increases down the group. General Chemistry Principles & Modern Applications. Is effective nuclear charge the same for all of the electrons present in an atom? In other words, the \(Z_{eff}\) calculated from Slater's rules are approximate values. Submit. Product was successfully added to your shopping cart. Anions are above the line; cations are below the line. A nuclear charge is equal to the electric charge of a nucleus of an atom. If there is only one electron in an orbit there will be no screening effect taking place. However, all electrons in the same orbit have the same effective nuclear charge. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Greatest --> Least The shielding constant for each group is formed as the sum of the following contributions: If the group (n) is of [s, p] type, an amount of 0.85 from each electron in (n-1)th shell and an amount of 1.00 for each electron from (n-2) and lower shells is added to the shielding constant. Down the table: As we go down a column of the periodic table, the valence \(Z_{eff}\) increases. Therefore, the electrons in oxygen are held closer to the nucleus, giving it a smaller radius. However, one might think that since the number of valence electrons increase going down the group, the element should be more stable and have higher electron affinity. Te The presence of multiple electrons results in more repulsive force and this decreases the nuclear attraction to some extent. Rules for electrons to the left of the group containing electron of interest: If the electron-of-interest is in a d or f subshell, then all electrons in groups to the left of the group having electron-of-interest contribute 1.00 to the shielding constant. Identification of the dagger/mini sword which has been in my family for as long as I can remember (and I am 80 years old), SSD has SMART test PASSED but fails self-testing. This will be always less than the actual nuclear charge due to the shielding effect. What is the ground state electronic configuration of Ti+4? Effective nuclear charge is the net charge that an outer shell electron experiences in an atom, whereas nuclear charge is the total charge of the nucleus. The best answers are voted up and rise to the top, Not the answer you're looking for? You dont consider f orbitals for bromine either. This page titled 1.1.2: Effective Nuclear Charge is shared under a not declared license and was authored, remixed, and/or curated by Kathryn Haas. Thanks to all authors for creating a page that has been read 307,443 times. Explain how and why ionization energy depends on \(Z_{eff}\). A fluorine atom has an electronic structure of 1s22s22px22py22pz1. It is useful to understand trends in valence \(Z_{eff}\) because the valence \(Z_{eff}\) determines atomic/ionic properties and chemical reactivity. As we go across periods 1-3, the shell remains constant as Z increases and the subshell changes from s to p. In these periods, there is a gradual increase in valence Zeff. Which of the following metals might have been the element studied? Prentice Hall. 1. Which group is likely to react with chlorine to form compounds in the form XCl? wikiHow is a wiki, similar to Wikipedia, which means that many of our articles are co-written by multiple authors. Each outer electron in effect feels a pull of 7+ from the center of the atom, irrespective of which element you are talking about. The ionization energy for lithium is 520 kJ/molkJ/mol. Ionisation energy is the energy required to remove a valence electron from an atom. As you move down a group of the periodic table, does electron affinity increase or decrease, if so, why? { Atomic_and_Ionic_Radius : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
