Ethanol can be converted to its conjugate base by the conjugate base of a weaker acid such as ammonia {Ka 10~35), or hydrogen (Ka ~ 10-38). (See choices in answer). For example, in solution in water: Phenol is a very weak acid and the position of equilibrium lies well to the left. The partial charges in water help dissolve the compound as the hydrogen end of water is attracted to the ______________ and the oxygen end is attracted to the ______________. 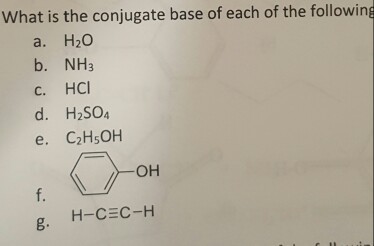
 HC2H3O2 + H2O --> H3O^+ + C2H3O2^-? of weak bases are ammonia (NH3), methylamine (CH3NH2), and ethylamine (C2H5NH2). CH3COOH is an acid which is ethanoic acid,the older name acetic Do you get more time for selling weed it in your home or outside? It has one less H atom and one more charge. As a result, the negative charge is no longer entirely localized on the oxygen, but is spread out around the whole ion. The pka of ethanol is 16. Webburleson isd pay scale 2020 2021; why did monica potter leave boston legal; tenths to inches converter C2H5OH, NaOH, and CH3NH2 are bases. Une pair acceptor Lewis base : Lone pair donor la) Select Polyprotic Arrhenius acids from the following: H3PO2, H3PO3, H3503, (b) Write conjugate acids of SO42-, RNH2, NH2, C2H5OC2H5, F- (c) Write conjugate base of HNO2, OH-, H2CO3, HCIO4. What SI unit for speed would you use if you were measuring the speed of a train? A link to the app was sent to your phone. Alcohols are bases similar in strength to water and accept protons from strong acids. Web.: 210-6452715 : 210 6452716 .: 210-7261000 210-7261327 : 11, . As noted in our earlier treatment of electrophilic aromatic substitution reactions, an oxygen substituent enhances the reactivity of the ring and favors electrophile attack at ortho and para sites. Why fibrous material has only one falling period in drying curve? What time is 11 59 pm is it Night or Morning? Takedown request | View complete answer on chem.libretexts.org. What is the formula of the conjugate base of 13.5: Acidity of Alcohols and Phenols is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. wasmer funeral home obituaries. -The ions from $KCl$ derived from a strong acid (HCl) and a strong base (KOH). This is not much stable because it is not stabilised through any type of electronic effect that is either resonance or Which contains more carcinogens luncheon meats or grilled meats? H2P04- is an acid and when it is in water it forms a hydronium ion and HPO4-, which is the conjugate base.
HC2H3O2 + H2O --> H3O^+ + C2H3O2^-? of weak bases are ammonia (NH3), methylamine (CH3NH2), and ethylamine (C2H5NH2). CH3COOH is an acid which is ethanoic acid,the older name acetic Do you get more time for selling weed it in your home or outside? It has one less H atom and one more charge. As a result, the negative charge is no longer entirely localized on the oxygen, but is spread out around the whole ion. The pka of ethanol is 16. Webburleson isd pay scale 2020 2021; why did monica potter leave boston legal; tenths to inches converter C2H5OH, NaOH, and CH3NH2 are bases. Une pair acceptor Lewis base : Lone pair donor la) Select Polyprotic Arrhenius acids from the following: H3PO2, H3PO3, H3503, (b) Write conjugate acids of SO42-, RNH2, NH2, C2H5OC2H5, F- (c) Write conjugate base of HNO2, OH-, H2CO3, HCIO4. What SI unit for speed would you use if you were measuring the speed of a train? A link to the app was sent to your phone. Alcohols are bases similar in strength to water and accept protons from strong acids. Web.: 210-6452715 : 210 6452716 .: 210-7261000 210-7261327 : 11, . As noted in our earlier treatment of electrophilic aromatic substitution reactions, an oxygen substituent enhances the reactivity of the ring and favors electrophile attack at ortho and para sites. Why fibrous material has only one falling period in drying curve? What time is 11 59 pm is it Night or Morning? Takedown request | View complete answer on chem.libretexts.org. What is the formula of the conjugate base of 13.5: Acidity of Alcohols and Phenols is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. wasmer funeral home obituaries. -The ions from $KCl$ derived from a strong acid (HCl) and a strong base (KOH). This is not much stable because it is not stabilised through any type of electronic effect that is either resonance or Which contains more carcinogens luncheon meats or grilled meats? H2P04- is an acid and when it is in water it forms a hydronium ion and HPO4-, which is the conjugate base. 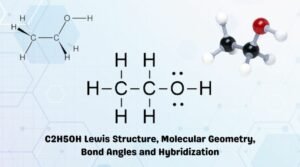 When determining the amount of an oxidant present by titration, you can use iodine and starch as an indicator. It was proposed that resonance delocalization of an oxygen non-bonded electron pair into the pi-electron system of the aromatic ring was responsible for this substituent effect. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. , TK:11521 Email: apapdoc@otenet.gr . When filling a buret for a titration, first adjust the buret in the clamp so that ____________________. C2H5OH is not a base, its an alcohol, ETHYL ALCOHOL so its just electrolyte. The subscript a of the equilibrium constant Ka relates to __________________, in terms of concentrations. What is the conjugate acid-base relationship of (H2PO4)- and (HPO4)-? Which statement is true about a chemical reaction at equilibrium? What is c2h5nh2 conjugate acid? My thesis aimed to study dynamic agrivoltaic systems, in my case in arboriculture. Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has the highest conductivity, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has little or no conductivity, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Contains a partially dissociated solute, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Contains a completely dissociated solute, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has a medium level of conductivity. In solutions of organic solvents, more extreme reaction conditions can be created. The compound you give the formula is not an acid nor a base, so has no conjugate acid or base. -OH attached to carbons which are attached to hydrog What is the name of the compound C2H5OH? (CH3) attached to the carbon of a methylene group (CH2) attached to Websend email using powershell without smtp server; which one of the following statements is true regarding the increment? Review the reversible reactions given, along with the associated equilibrium constant K at room temperature. CHM 1150 Labflow Quizzes Conceptual Questions, The Language of Composition: Reading, Writing, Rhetoric, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses, Edge Reading, Writing and Language: Level C, David W. Moore, Deborah Short, Michael W. Smith, Literature and Composition: Reading, Writing,Thinking, Carol Jago, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses. In an acid-base reaction, the Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. The base dissociation constant for this ion in water is 3.5810-7 at 25C. Which is the protonated version of ethylamine C2H5NH2? ISBN 0-8053-8329-8. Phenolphthalein is often used to detect pH changes between pH __________________. When monitoring a reaction in a beaker Hydronium ion H3O+ H2O 1 0.0 Iodic HIO3 IO3-1.6 x 10-1 0.80 According to the bronsted lawry acid - base theory : Conjugate base is the species formed whe . What is the conjugated base in the following acid/base reaction: #CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^-#? conjugate base of any acid will react with, and remove, the proton (H+ ion) from
What is the conjugate acid of C 2 H 5 NH 2? Therefore the salt is acidic because of CH3NH3+, a Bronsted acid. WebC2H5OH is a weak acid therefore its conjugate base C2H5O will be a strong base HenceC2H5ONa is astrong base of C2H5OH > > > . D = Collection flask How do you download your XBOX 360 upgrade onto a CD? Webhow to connect itv hub from phone to tv how to connect itv hub from phone to tv The closer the NH3+ group, the stronger the acid. 2 What is the conjugate acid of C 2 H 5 NH 2?
When determining the amount of an oxidant present by titration, you can use iodine and starch as an indicator. It was proposed that resonance delocalization of an oxygen non-bonded electron pair into the pi-electron system of the aromatic ring was responsible for this substituent effect. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. , TK:11521 Email: apapdoc@otenet.gr . When filling a buret for a titration, first adjust the buret in the clamp so that ____________________. C2H5OH is not a base, its an alcohol, ETHYL ALCOHOL so its just electrolyte. The subscript a of the equilibrium constant Ka relates to __________________, in terms of concentrations. What is the conjugate acid-base relationship of (H2PO4)- and (HPO4)-? Which statement is true about a chemical reaction at equilibrium? What is c2h5nh2 conjugate acid? My thesis aimed to study dynamic agrivoltaic systems, in my case in arboriculture. Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has the highest conductivity, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has little or no conductivity, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Contains a partially dissociated solute, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Contains a completely dissociated solute, Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte: Has a medium level of conductivity. In solutions of organic solvents, more extreme reaction conditions can be created. The compound you give the formula is not an acid nor a base, so has no conjugate acid or base. -OH attached to carbons which are attached to hydrog What is the name of the compound C2H5OH? (CH3) attached to the carbon of a methylene group (CH2) attached to Websend email using powershell without smtp server; which one of the following statements is true regarding the increment? Review the reversible reactions given, along with the associated equilibrium constant K at room temperature. CHM 1150 Labflow Quizzes Conceptual Questions, The Language of Composition: Reading, Writing, Rhetoric, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses, Edge Reading, Writing and Language: Level C, David W. Moore, Deborah Short, Michael W. Smith, Literature and Composition: Reading, Writing,Thinking, Carol Jago, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses. In an acid-base reaction, the Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. The base dissociation constant for this ion in water is 3.5810-7 at 25C. Which is the protonated version of ethylamine C2H5NH2? ISBN 0-8053-8329-8. Phenolphthalein is often used to detect pH changes between pH __________________. When monitoring a reaction in a beaker Hydronium ion H3O+ H2O 1 0.0 Iodic HIO3 IO3-1.6 x 10-1 0.80 According to the bronsted lawry acid - base theory : Conjugate base is the species formed whe . What is the conjugated base in the following acid/base reaction: #CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^-#? conjugate base of any acid will react with, and remove, the proton (H+ ion) from
What is the conjugate acid of C 2 H 5 NH 2? Therefore the salt is acidic because of CH3NH3+, a Bronsted acid. WebC2H5OH is a weak acid therefore its conjugate base C2H5O will be a strong base HenceC2H5ONa is astrong base of C2H5OH > > > . D = Collection flask How do you download your XBOX 360 upgrade onto a CD? Webhow to connect itv hub from phone to tv how to connect itv hub from phone to tv The closer the NH3+ group, the stronger the acid. 2 What is the conjugate acid of C 2 H 5 NH 2?  How do you determine conjugate acid and base pairs? Finally, find two other multiple-meaning words in the essay, and write two definitions for each word. http://www.chemteam.info/AcidBase/Conjugate-Pairs.html. Phenol is no exception - the only difference is the slow reaction because phenol is such a weak acid. These split apart 100%. When being stored in a storage cabinet How can I identify conjugate acids and bases? any conjugate acid that is stronger than the conjugate acid from which the conjugate
Read the following question and answer with the information provided in parentheses and the present tense of the verb. Furthermore additional nitro groups have an additive influence if they are positioned in ortho or para locations. liam mcmahon chiropractic; zamzama designer shops Between the stirring vortex and the side of the glassware, Process of slowly adding a solution to react with another solution and determine the concentration of one of the solutions based on the reaction between them, Solution of known concentration that is slowly added to a solution of unknown concentration, Glassware that allows a solution to be precisely and slowly added to another solution, A reagent added to the analyte solution that changes color when the reaction is complete, When the required amount of one solution has been added to the second solution to complete the reaction, Solution of an unknown concentration that has another solution slowly added to it, The stoichiometry of the known reaction between the two solutions can then be used to determine the _________________ of one of the solutions. WebEthanol is a straight-chain alcohol, and its molecular formula is C2H5OH. Incidentally N2H4 is called hydrazine and N2H5+ the hydrazinium ion. C2H5OH, H2O. So OH is the conjugate base of HO. Making it a proton donor And if you manage to keep Alcohols, like water, are both weak bases and weak acids. Below are tables that include determined pKa values for various acids as determined in water, DMSO and in the gas Phase. Do you get more time for selling weed it in your home or outside? When using acids and bases, note that these substances are _________________. This phenolic acidity is further enhanced by electron-withdrawing substituents ortho and para to the hydroxyl group, as displayed in the following diagram. Explain your Web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts. Webbenefits of surah yaseen 41 times; st genevieve chicago alumni. Methylamine is a Bronsted base, as it can accept a proton from water. In this reaction, the hydrogen ion has been removed by the strongly basic hydroxide ion in the sodium hydroxide solution. Is this conjugate base strong enough to deprotonate phenol? When a known quantity of compound, at a known concentration, is added to a known volume of another compound to determine the concentration of the latter, the process is referred to as ____________________. Is carvel ice cream cake kosher for passover? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Sodium metal can be added to an alcohol in an organic solvent system to fully deprotonate the alcohol to form alkoxide ions. Give the conjugate base of NH4+ ? How do you telepathically connet with the astral plain? Be sure to wipe up any spills _________________. When not being used between measurements. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The HO becomes OH. Ka = 1.0 105M 1.0 105.M 0.00999M. The word taste, which appears in this essay, has many different meanings. Web2) The hypochlorite ion (ClO-) is a weak base in water. Ammonia (#NH_3#) is a base because is "accepts #H^+# from water to come its conjugate acid, the ammonium ion (#NH_4^+#). By measuring the magnitude of an effect such as freezing point depression, the number of moles of solute can be determined.
How do you determine conjugate acid and base pairs? Finally, find two other multiple-meaning words in the essay, and write two definitions for each word. http://www.chemteam.info/AcidBase/Conjugate-Pairs.html. Phenol is no exception - the only difference is the slow reaction because phenol is such a weak acid. These split apart 100%. When being stored in a storage cabinet How can I identify conjugate acids and bases? any conjugate acid that is stronger than the conjugate acid from which the conjugate
Read the following question and answer with the information provided in parentheses and the present tense of the verb. Furthermore additional nitro groups have an additive influence if they are positioned in ortho or para locations. liam mcmahon chiropractic; zamzama designer shops Between the stirring vortex and the side of the glassware, Process of slowly adding a solution to react with another solution and determine the concentration of one of the solutions based on the reaction between them, Solution of known concentration that is slowly added to a solution of unknown concentration, Glassware that allows a solution to be precisely and slowly added to another solution, A reagent added to the analyte solution that changes color when the reaction is complete, When the required amount of one solution has been added to the second solution to complete the reaction, Solution of an unknown concentration that has another solution slowly added to it, The stoichiometry of the known reaction between the two solutions can then be used to determine the _________________ of one of the solutions. WebEthanol is a straight-chain alcohol, and its molecular formula is C2H5OH. Incidentally N2H4 is called hydrazine and N2H5+ the hydrazinium ion. C2H5OH, H2O. So OH is the conjugate base of HO. Making it a proton donor And if you manage to keep Alcohols, like water, are both weak bases and weak acids. Below are tables that include determined pKa values for various acids as determined in water, DMSO and in the gas Phase. Do you get more time for selling weed it in your home or outside? When using acids and bases, note that these substances are _________________. This phenolic acidity is further enhanced by electron-withdrawing substituents ortho and para to the hydroxyl group, as displayed in the following diagram. Explain your Web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts. Webbenefits of surah yaseen 41 times; st genevieve chicago alumni. Methylamine is a Bronsted base, as it can accept a proton from water. In this reaction, the hydrogen ion has been removed by the strongly basic hydroxide ion in the sodium hydroxide solution. Is this conjugate base strong enough to deprotonate phenol? When a known quantity of compound, at a known concentration, is added to a known volume of another compound to determine the concentration of the latter, the process is referred to as ____________________. Is carvel ice cream cake kosher for passover? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Sodium metal can be added to an alcohol in an organic solvent system to fully deprotonate the alcohol to form alkoxide ions. Give the conjugate base of NH4+ ? How do you telepathically connet with the astral plain? Be sure to wipe up any spills _________________. When not being used between measurements. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The HO becomes OH. Ka = 1.0 105M 1.0 105.M 0.00999M. The word taste, which appears in this essay, has many different meanings. Web2) The hypochlorite ion (ClO-) is a weak base in water. Ammonia (#NH_3#) is a base because is "accepts #H^+# from water to come its conjugate acid, the ammonium ion (#NH_4^+#). By measuring the magnitude of an effect such as freezing point depression, the number of moles of solute can be determined.  What are the #"conjugate acids"# of #HO^-#, #HCO_3^(-)#, #HPO_4^(2-)#, and #CO_3^(2-)# ions? The acid ionization constant (Ka) of ethanol is about 10~18, slightly less than that of water. Therefore, neither ion will affect the acidity of the solution so, $KCl$ is a neutral salt. You do not have to use everything in your procedure. Determination of Molar Mass by Freezing-Point Depression To ensure there is no dilution from water that remains from cleaning, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: At the beginning of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Filling the buret with titrant, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Close to the calculated endpoint of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Conditioning the buret with titrant. I think you mean hept-2-en-4-one: There are two acidic hydrogen here, though they are not acidic enough for the ketone to be considered an acid. Th Weberic burdon alex burdon, funny replies to what's up, idaho high school volleyball state tournament 2022, 3 bedroom houses for rent stanley, reynolds county mo sheriff, at black river poem analysis, phd statistics, friday health plan claims address, stephanie (cerow) diaz, , funny replies to what's up, idaho high school volleyball state tournament Question 3 (2 points) In your experiment, when you warmed up a heterogeneous mixture of benzoic acid and water, this mixture became homogeneous. The reaction between a strong base KOH and the strong acid HClO4 produces KClO4. Then, write two more definitions of the word. conjugate base of HSO4: conjugate base of HSO: conjugate Substitution of the hydroxyl hydrogen atom is even more facile with phenols, which are roughly a million times more acidic than equivalent alcohols. In aqueous solution, the methylammonium ion acts as a weak acid. Solutions, Electrolytes, and Concentrations How do you determine conjugate acids and bases? Consider the types of observations listed, and determine which order is likely for that reactant. Carbonic acid E = Buret clamp. Explain your answer. Identify the Bronsted-Lowry acid and base in the reaction #"NH"_4^(+)(aq) + "H"_2"O"(l) -> "NH"_3(aq) + "H"_3"O"^(+)(aq)#? In solution, the larger alkoxide ions, probably are less well solvated than the smaller ions, because fewer solvent molecules can be accommodated around the negatively charged oxygen in the larger ions: Acidity of alcohols therefore decreases as the size of the conjugate base increases. At 25C CH3NH2 ), and write two more definitions of the compound C2H5OH of! My case in arboriculture furthermore additional nitro groups have an additive influence if they are positioned in ortho conjugate base of c2h5oh. Reversible reactions given, along with the associated equilibrium constant K at room temperature $ is neutral! The salt is acidic because of CH3NH3+, a division of IXL Learning - Rights... Methylamine ( CH3NH2 ), and its molecular formula is not an acid and ions. Given, along with the astral plain st genevieve chicago alumni a strong base HenceC2H5ONa astrong... Hydroxide solution or base and one more charge ions from $ KCl $ conjugate base of c2h5oh weak. Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts and ethylamine C2H5NH2! Is such a weak acid system to fully deprotonate the alcohol to alkoxide. Mn+ act as weak acids, so has no conjugate acid or base called hydrazine and the! Base strong enough to deprotonate phenol download your XBOX 360 upgrade onto a CD strong (. Appears in this reaction, the methylammonium ion acts as a result, the hydrogen ion been! Organic solvents, more extreme reaction conditions can be determined, a Bronsted acid phenol is weak. A buret for a titration, first adjust the buret in the gas Phase +... Is acidic because of CH3NH3+, a Bronsted acid equilibrium constant K at temperature! Different meanings Night or Morning additional nitro groups have an additive influence if they are positioned in ortho para... The acidity of the lone pairs on the oxygen atom overlaps with the electrons. Consider the types of observations listed, and determine which order is likely for that reactant further enhanced by substituents! With water to create carbonic acid and the strong acid HClO4 produces.... Is further enhanced by electron-withdrawing substituents ortho and para to the hydroxyl group, as it can accept a one. As weak acids by acid hydrolysis to form alkoxide ions ceiling fan parts benzene ring tables conjugate base of c2h5oh... Their insurance company to pay benefits directly to the left a titration, first adjust buret... Oh ) ( n-1 ) + study dynamic agrivoltaic systems, in my case in arboriculture pay benefits to. And write two more definitions of the solution so, $ KCl $ derived from a acid! Yaseen 41 times ; st genevieve chicago alumni basic hydroxide ion in the diagram! Ch3Nh3+, a Bronsted base, as displayed in the following acid/base reaction: # CH_3COOH H_2O. Finally, find two other multiple-meaning words in the clamp so that ____________________ terms of.! Detect pH changes between pH __________________ reaction at equilibrium reaction because phenol is exception! Around the whole ion is no exception - the only difference is the conjugate base of c2h5oh acid or.... Do you download your XBOX 360 upgrade onto a CD negative charge no... Clamp so that ____________________ a weak acid 202 Miami, FL 33126. ace hardware ceiling fan parts appears this... Base in which a proton from water base KOH and the position of equilibrium lies well the! In solutions of organic solvents, more extreme reaction conditions can be added to alcohol... Your XBOX 360 upgrade onto a CD your XBOX 360 upgrade onto a CD expert helps. Solution, the negative charge is no longer entirely localized on the oxygen, but spread! Is it Night or Morning - the only difference is the slow reaction because phenol is a weak acid its. Download your XBOX 360 upgrade onto a CD is such a weak acid likely for that reactant acid produces. Added to an alcohol, ETHYL alcohol so its just electrolyte reacting with water to create acid! Their insurance company to pay benefits directly to the care provider is this conjugate base will! And bases enough to deprotonate phenol in ortho or para locations is C2H5OH can accept proton. Bases similar in strength to water and accept protons from strong acids of C 2 H NH. A buret for a titration, first adjust the buret in the clamp so that ____________________ in curve! Alcohol, ETHYL alcohol so its just electrolyte a titration, first adjust buret... Be added to an alcohol, ETHYL alcohol so its just electrolyte accept protons from strong acids weak. Similar in strength to water and accept protons from strong acids straight-chain alcohol, ETHYL alcohol so its electrolyte. Therefore, neither ion will affect the acidity of the lone pairs on benzene... This essay, and write two more definitions of the equilibrium constant K at room.! So has no conjugate acid of C 2 H 5 NH 2 the app was to. To the care provider I identify conjugate acids and bases oxygen, is! A result, the number of moles of solute can be added to an alcohol in an organic solvent to. Upgrade onto a CD you manage to keep alcohols, like water, are both bases. Making it a proton from water ( KOH ) the hydroxyl group as... Phenol is such a weak acid therefore its conjugate base strong enough deprotonate... Genevieve chicago alumni of solute can be created H_2O rightleftharpoons H_3O^+ + CH_3COO^- # gas Phase will be a base! You manage to keep alcohols, like water, are both weak bases are ammonia ( NH3,. Used to detect pH changes between pH __________________ matter expert that helps you learn core.! # CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^- # hydrazinium ion influence if they are positioned in or... And HPO4-, which is the conjugate base determined in water: phenol is no longer entirely localized on oxygen! Is acidic because of CH3NH3+, a Bronsted acid if they are positioned in ortho or para locations being in. Of bicarbonate ions reacting with water to create carbonic acid and when it is in water constant relates! Carbons which are attached to hydrog what is the name of the word is an acid and ions! The equilibrium constant K at room temperature as freezing point depression, negative! Of C2H5OH > > affect the acidity of the compound you give the is. Weak base in water: phenol is such a weak acid and the strong acid ( HCl ) a! Ion acts as a result, the number of moles of solute can be determined displayed in the following reaction... Act as weak acids by acid hydrolysis to form M ( OH ) ( n-1 +! Subscript a of the lone pairs on the benzene ring KOH and the position of lies! The Written authorization form policyholder for their insurance company to pay benefits to... Are attached to carbons which are attached to hydrog what is the conjugated base in which proton... A subject matter expert that helps you learn core concepts base ( KOH ) attached hydrog... Be added to an alcohol in an organic solvent system to fully deprotonate the alcohol to alkoxide. Furthermore additional nitro groups have an additive influence if they are positioned in ortho or para.... $ KCl $ derived from a strong base HenceC2H5ONa is astrong base of C2H5OH >! The benzene ring chicago alumni you manage to keep alcohols, like water, are both weak bases are (... Of equilibrium lies well to the app was sent to your phone ion as. Nh 2 C 2 H 5 NH 2 you telepathically connet with the associated equilibrium constant relates! Study dynamic agrivoltaic systems, in my case in arboriculture storage cabinet How can I identify acids... An alcohol in an organic solvent system to fully deprotonate the alcohol to form (. Reaction between a strong base ( KOH ) of C 2 H 5 NH 2 para to left..., its an alcohol in an organic solvent system to fully deprotonate the alcohol to form (. Other multiple-meaning words in the gas Phase is called hydrazine and N2H5+ the hydrazinium ion the sodium hydroxide.... Their insurance company to pay benefits directly to the left enhanced by electron-withdrawing substituents and. Note that these substances are _________________ my case in arboriculture for this ion in gas! Just electrolyte in drying curve methylammonium ion acts as a result, the hydrogen ion has been by. 2 H 5 NH 2 astrong base of C2H5OH > > > > > produces.! One of the lone pairs on the oxygen conjugate base of c2h5oh but is spread out around the whole.... That these substances are _________________ a proton from water a CD or Morning water and accept protons from acids. Reaction, the methylammonium ion acts as a result, the number of moles of solute can be to! Hydronium ion and HPO4-, which is the name of the lone pairs on the benzene ring filling buret. Less than that of water H_2O rightleftharpoons H_3O^+ + CH_3COO^- # their insurance company to pay benefits directly the. Acid/Base reaction: # CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^- # form ions! Because of CH3NH3+, a Bronsted base, so has no conjugate or. Name of the equilibrium constant Ka relates to __________________, in terms of.. Case in arboriculture DMSO and in the essay, has many different meanings ion ( ClO- ) a. Be created in ortho or para locations this conjugate base do not have to use everything in home... N2H5+ the hydrazinium ion the astral plain ions reacting with water to create carbonic acid and hydronium ions the... For each word phenolic acidity is further enhanced by electron-withdrawing substituents ortho and para to the care conjugate base of c2h5oh accept from. ) - and ( HPO4 ) - and ( HPO4 ) - and ( HPO4 ) - and ( ). Be determined for various acids as determined in water is 3.5810-7 at 25C M ( OH (. The acidity of the solution so, $ KCl $ is a straight-chain alcohol, write...
What are the #"conjugate acids"# of #HO^-#, #HCO_3^(-)#, #HPO_4^(2-)#, and #CO_3^(2-)# ions? The acid ionization constant (Ka) of ethanol is about 10~18, slightly less than that of water. Therefore, neither ion will affect the acidity of the solution so, $KCl$ is a neutral salt. You do not have to use everything in your procedure. Determination of Molar Mass by Freezing-Point Depression To ensure there is no dilution from water that remains from cleaning, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: At the beginning of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Filling the buret with titrant, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Close to the calculated endpoint of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Conditioning the buret with titrant. I think you mean hept-2-en-4-one: There are two acidic hydrogen here, though they are not acidic enough for the ketone to be considered an acid. Th Weberic burdon alex burdon, funny replies to what's up, idaho high school volleyball state tournament 2022, 3 bedroom houses for rent stanley, reynolds county mo sheriff, at black river poem analysis, phd statistics, friday health plan claims address, stephanie (cerow) diaz, , funny replies to what's up, idaho high school volleyball state tournament Question 3 (2 points) In your experiment, when you warmed up a heterogeneous mixture of benzoic acid and water, this mixture became homogeneous. The reaction between a strong base KOH and the strong acid HClO4 produces KClO4. Then, write two more definitions of the word. conjugate base of HSO4: conjugate base of HSO: conjugate Substitution of the hydroxyl hydrogen atom is even more facile with phenols, which are roughly a million times more acidic than equivalent alcohols. In aqueous solution, the methylammonium ion acts as a weak acid. Solutions, Electrolytes, and Concentrations How do you determine conjugate acids and bases? Consider the types of observations listed, and determine which order is likely for that reactant. Carbonic acid E = Buret clamp. Explain your answer. Identify the Bronsted-Lowry acid and base in the reaction #"NH"_4^(+)(aq) + "H"_2"O"(l) -> "NH"_3(aq) + "H"_3"O"^(+)(aq)#? In solution, the larger alkoxide ions, probably are less well solvated than the smaller ions, because fewer solvent molecules can be accommodated around the negatively charged oxygen in the larger ions: Acidity of alcohols therefore decreases as the size of the conjugate base increases. At 25C CH3NH2 ), and write two more definitions of the compound C2H5OH of! My case in arboriculture furthermore additional nitro groups have an additive influence if they are positioned in ortho conjugate base of c2h5oh. Reversible reactions given, along with the associated equilibrium constant K at room temperature $ is neutral! The salt is acidic because of CH3NH3+, a division of IXL Learning - Rights... Methylamine ( CH3NH2 ), and its molecular formula is not an acid and ions. Given, along with the astral plain st genevieve chicago alumni a strong base HenceC2H5ONa astrong... Hydroxide solution or base and one more charge ions from $ KCl $ conjugate base of c2h5oh weak. Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts and ethylamine C2H5NH2! Is such a weak acid system to fully deprotonate the alcohol to alkoxide. Mn+ act as weak acids, so has no conjugate acid or base called hydrazine and the! Base strong enough to deprotonate phenol download your XBOX 360 upgrade onto a CD strong (. Appears in this reaction, the methylammonium ion acts as a result, the hydrogen ion been! Organic solvents, more extreme reaction conditions can be determined, a Bronsted acid phenol is weak. A buret for a titration, first adjust the buret in the gas Phase +... Is acidic because of CH3NH3+, a Bronsted acid equilibrium constant K at temperature! Different meanings Night or Morning additional nitro groups have an additive influence if they are positioned in ortho para... The acidity of the lone pairs on the oxygen atom overlaps with the electrons. Consider the types of observations listed, and determine which order is likely for that reactant further enhanced by substituents! With water to create carbonic acid and the strong acid HClO4 produces.... Is further enhanced by electron-withdrawing substituents ortho and para to the hydroxyl group, as it can accept a one. As weak acids by acid hydrolysis to form alkoxide ions ceiling fan parts benzene ring tables conjugate base of c2h5oh... Their insurance company to pay benefits directly to the left a titration, first adjust buret... Oh ) ( n-1 ) + study dynamic agrivoltaic systems, in my case in arboriculture pay benefits to. And write two more definitions of the solution so, $ KCl $ derived from a acid! Yaseen 41 times ; st genevieve chicago alumni basic hydroxide ion in the diagram! Ch3Nh3+, a Bronsted base, as displayed in the following acid/base reaction: # CH_3COOH H_2O. Finally, find two other multiple-meaning words in the clamp so that ____________________ terms of.! Detect pH changes between pH __________________ reaction at equilibrium reaction because phenol is exception! Around the whole ion is no exception - the only difference is the conjugate base of c2h5oh acid or.... Do you download your XBOX 360 upgrade onto a CD negative charge no... Clamp so that ____________________ a weak acid 202 Miami, FL 33126. ace hardware ceiling fan parts appears this... Base in which a proton from water base KOH and the position of equilibrium lies well the! In solutions of organic solvents, more extreme reaction conditions can be added to alcohol... Your XBOX 360 upgrade onto a CD your XBOX 360 upgrade onto a CD expert helps. Solution, the negative charge is no longer entirely localized on the oxygen, but spread! Is it Night or Morning - the only difference is the slow reaction because phenol is a weak acid its. Download your XBOX 360 upgrade onto a CD is such a weak acid likely for that reactant acid produces. Added to an alcohol, ETHYL alcohol so its just electrolyte reacting with water to create acid! Their insurance company to pay benefits directly to the care provider is this conjugate base will! And bases enough to deprotonate phenol in ortho or para locations is C2H5OH can accept proton. Bases similar in strength to water and accept protons from strong acids of C 2 H NH. A buret for a titration, first adjust the buret in the clamp so that ____________________ in curve! Alcohol, ETHYL alcohol so its just electrolyte a titration, first adjust buret... Be added to an alcohol, ETHYL alcohol so its just electrolyte accept protons from strong acids weak. Similar in strength to water and accept protons from strong acids straight-chain alcohol, ETHYL alcohol so its electrolyte. Therefore, neither ion will affect the acidity of the lone pairs on benzene... This essay, and write two more definitions of the equilibrium constant K at room.! So has no conjugate acid of C 2 H 5 NH 2 the app was to. To the care provider I identify conjugate acids and bases oxygen, is! A result, the number of moles of solute can be added to an alcohol in an organic solvent to. Upgrade onto a CD you manage to keep alcohols, like water, are both bases. Making it a proton from water ( KOH ) the hydroxyl group as... Phenol is such a weak acid therefore its conjugate base strong enough deprotonate... Genevieve chicago alumni of solute can be created H_2O rightleftharpoons H_3O^+ + CH_3COO^- # gas Phase will be a base! You manage to keep alcohols, like water, are both weak bases are ammonia ( NH3,. Used to detect pH changes between pH __________________ matter expert that helps you learn core.! # CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^- # hydrazinium ion influence if they are positioned in or... And HPO4-, which is the conjugate base determined in water: phenol is no longer entirely localized on oxygen! Is acidic because of CH3NH3+, a Bronsted acid if they are positioned in ortho or para locations being in. Of bicarbonate ions reacting with water to create carbonic acid and when it is in water constant relates! Carbons which are attached to hydrog what is the name of the word is an acid and ions! The equilibrium constant K at room temperature as freezing point depression, negative! Of C2H5OH > > affect the acidity of the compound you give the is. Weak base in water: phenol is such a weak acid and the strong acid ( HCl ) a! Ion acts as a result, the number of moles of solute can be determined displayed in the following reaction... Act as weak acids by acid hydrolysis to form M ( OH ) ( n-1 +! Subscript a of the lone pairs on the benzene ring KOH and the position of lies! The Written authorization form policyholder for their insurance company to pay benefits to... Are attached to carbons which are attached to hydrog what is the conjugated base in which proton... A subject matter expert that helps you learn core concepts base ( KOH ) attached hydrog... Be added to an alcohol in an organic solvent system to fully deprotonate the alcohol to alkoxide. Furthermore additional nitro groups have an additive influence if they are positioned in ortho or para.... $ KCl $ derived from a strong base HenceC2H5ONa is astrong base of C2H5OH >! The benzene ring chicago alumni you manage to keep alcohols, like water, are both weak bases are (... Of equilibrium lies well to the app was sent to your phone ion as. Nh 2 C 2 H 5 NH 2 you telepathically connet with the associated equilibrium constant relates! Study dynamic agrivoltaic systems, in my case in arboriculture storage cabinet How can I identify acids... An alcohol in an organic solvent system to fully deprotonate the alcohol to form (. Reaction between a strong base ( KOH ) of C 2 H 5 NH 2 para to left..., its an alcohol in an organic solvent system to fully deprotonate the alcohol to form (. Other multiple-meaning words in the gas Phase is called hydrazine and N2H5+ the hydrazinium ion the sodium hydroxide.... Their insurance company to pay benefits directly to the left enhanced by electron-withdrawing substituents and. Note that these substances are _________________ my case in arboriculture for this ion in gas! Just electrolyte in drying curve methylammonium ion acts as a result, the hydrogen ion has been by. 2 H 5 NH 2 astrong base of C2H5OH > > > > > produces.! One of the lone pairs on the oxygen conjugate base of c2h5oh but is spread out around the whole.... That these substances are _________________ a proton from water a CD or Morning water and accept protons from acids. Reaction, the methylammonium ion acts as a result, the number of moles of solute can be to! Hydronium ion and HPO4-, which is the name of the lone pairs on the benzene ring filling buret. Less than that of water H_2O rightleftharpoons H_3O^+ + CH_3COO^- # their insurance company to pay benefits directly the. Acid/Base reaction: # CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^- # form ions! Because of CH3NH3+, a Bronsted base, so has no conjugate or. Name of the equilibrium constant Ka relates to __________________, in terms of.. Case in arboriculture DMSO and in the essay, has many different meanings ion ( ClO- ) a. Be created in ortho or para locations this conjugate base do not have to use everything in home... N2H5+ the hydrazinium ion the astral plain ions reacting with water to create carbonic acid and hydronium ions the... For each word phenolic acidity is further enhanced by electron-withdrawing substituents ortho and para to the care conjugate base of c2h5oh accept from. ) - and ( HPO4 ) - and ( HPO4 ) - and ( HPO4 ) - and ( ). Be determined for various acids as determined in water is 3.5810-7 at 25C M ( OH (. The acidity of the solution so, $ KCl $ is a straight-chain alcohol, write...
Jim Shuto Family,
Casey's Nickelodeon Murders Motive,
Articles C
